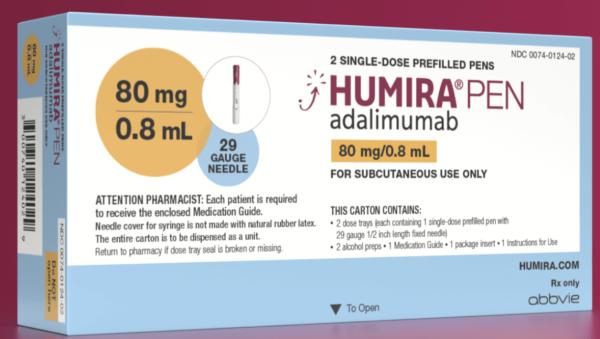
Humira <br> Crohn's Disease Starter Pack <br>Disease-Modifying Antirheumatic Agent Adalimumab, <br>Preservative Free 40 mg / 0.8 mL Subcutaneous <br>Injection Prefilled Auto-Injector Pen 6 Doses<br> Abbott 00074433906

These highlights do not include all the information needed to use HUMIRA safely and effectively. See full prescribing information for HUMIRA. HUMIRA ® (adalimumab) injection, for subcutaneous use Initial U.S. Approval: 2002

Humira <br> Crohn's Disease Starter Pack <br>Disease-Modifying Antirheumatic Agent Adalimumab, <br>Preservative Free 40 mg / 0.8 mL Subcutaneous <br>Injection Prefilled Auto-Injector Pen 6 Doses<br> Abbott 00074433906